Prenatal screening for genetic conditions has become a complex process with a multitude of tests to choose from. With advancing technology, the available options are becoming overwhelming for both health care professionals and patients. It can be difficult to understand all of the options and decide what is best for you. Below is a review the common prenatal screening options that are available along with the pertinent limitations and benefits of each.
It is important to remember that all of the following are OPTIONAL during a pregnancy. Prenatal screening for chromosome abnormalities should be made available to all women during all pregnancies (as recommended by the American Congress of Obstetricians and Gynecologists). Certainly there are women who choose not to have any prenatal screening done. Many women like to have the information provided by these tests to prepare for when the baby is born. Other women may use the information to guide decisions such as termination and adoption. In discussing these testing options I am not advocating for or protesting against the issue of abortion. My goal is to take a nondirective approach of explaining the commonly available prenatal screening options.
Topics Discussed
- What are Chromosome Abnormalities?
- Age Related Risk
- First Trimester Screening (the nuchal translucency test)
- Second Trimester Screening (the quad screen)
- Other Screening Options
- Noninvasive Prenatal Testing (Materniti21, Verifi, Panorama, Harmony)
- Diagnostic Testing
What Are Chromosome Abnormalities?
To understand what a chromosome abnormality is we have to take a step back and talk about some basic genetics.
Typically, we have 23 pairs of chromosomes in each cell of our body. A chromosome is made up of tightly wound DNA and can be thought of as a genetic blue print that determines everything from our hair color to our risk for genetic disease. The first 22 pairs of chromosomes are called autosomes and the last pair are the sex chromosome (XX for girls and XY for boys). For each chromosome pair, we inherit one chromosome from our mother and one chromosome from our father. During meiosis, a process of cell division, sex cells (sperm and eggs) are created that contain only one copy of each chromosome. This is important so that when the sperm fertilizes the egg there are a total of 46 chromosomes (half from mom and half from dad). However, sometimes errors are made in this process that can cause some of the sperm cells and egg cells to have extra or 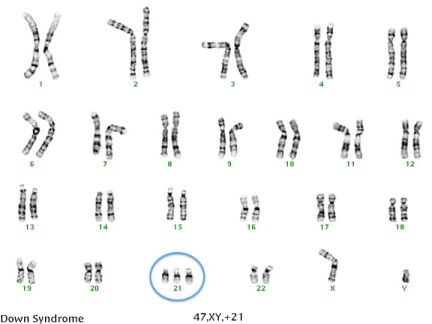 missing chromosomes. This is called nondisjunction. For example, if one of the sex cells has an extra chromosome 21, the resulting fetus will have three copies of chromosome 21 instead of two. This is called trisomy 21 (see image), but is more commonly referred to as Down syndrome. Down syndrome is a common chromosome abnormality, occurring in approximately 1 in every 800 recognized pregnancies. A trisomy can occur in any chromosome pair, but most will result in an early miscarriage before you even know you’re pregnant. Two other chromosomal trisomies that are sometimes compatible with life and those are trisomy 18 and trisomy 13. Both of these chromosome abnormalities are more severe than Down syndrome and are generally considered fatal conditions. Most pregnancies with trisomy 13 or 18 will result in a miscarriage. If the pregnancy makes it to term, the vast majority of babies won’t live past 1 year of life.
missing chromosomes. This is called nondisjunction. For example, if one of the sex cells has an extra chromosome 21, the resulting fetus will have three copies of chromosome 21 instead of two. This is called trisomy 21 (see image), but is more commonly referred to as Down syndrome. Down syndrome is a common chromosome abnormality, occurring in approximately 1 in every 800 recognized pregnancies. A trisomy can occur in any chromosome pair, but most will result in an early miscarriage before you even know you’re pregnant. Two other chromosomal trisomies that are sometimes compatible with life and those are trisomy 18 and trisomy 13. Both of these chromosome abnormalities are more severe than Down syndrome and are generally considered fatal conditions. Most pregnancies with trisomy 13 or 18 will result in a miscarriage. If the pregnancy makes it to term, the vast majority of babies won’t live past 1 year of life.
For a more detailed discussion of chromosomes and chromosome abnormalities check out this link.
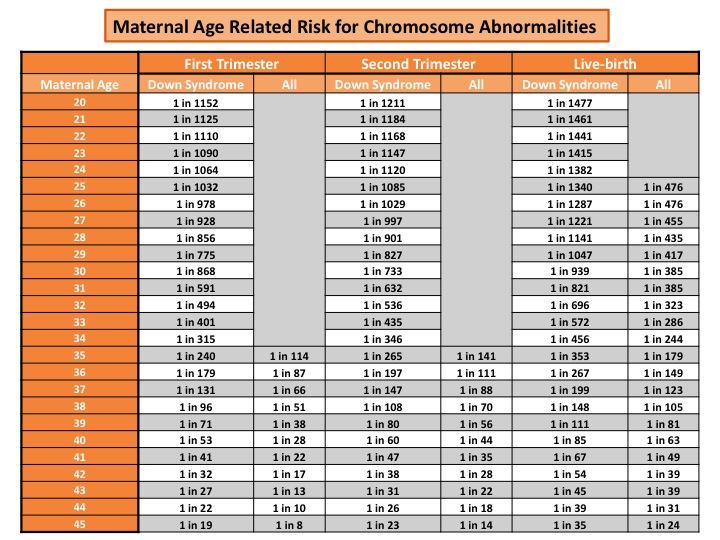
Before any prenatal testing is performed, a risk approximation for chromosome abnormalities can be given based on maternal age. As a woman get older, the risk for chromosome abnormalities in a pregnancy increases. Below is an example of a maternal age risk chart. It provides a specific risk for Down syndrome and a risk for all chromosomes abnormalities (which includes Down syndrome, trisomy 18, and trisomy 13). A mother’s risk decreases as the pregnancy progresses due to the risk of natural miscarriage.

First off, it’s important to know that a “screening” test will just provide you with a more accurate risk assessment. It cannot be used to diagnose or rule out a chromosome abnormality in a pregnancy. This is a first tier test that can either be reassuring or help guide decisions about potential follow-up testing.
The first part of the test involves an ultrasound that will measure the nuchal translucency (NT). The NT is an accumulation of fluid at the base of the baby’s neck that is expected to be seen in all pregnancies. A measurement above 3.0-3.5mm is considered to be “abnormal.” An increased NT is associated with a higher risk for chromosome abnormality or structural defect (like a heart defect).
The second part of the 1st trimester screen involves a blood draw from the mother that will measure two proteins that are made by the pregnancy (PAPP-A and hCG).
A risk assessment for the pregnancy will be calculated based on the NT measurement, protein levels from the blood draw, and numerous other factors (maternal age, ethinicity, diabetics, ect.). The detection rate for Down syndrome is 83% with a false positive rate of 5%. The detection rate for trisomy 18 is approximately 80% with a false positive rate of <1%.
The 1st trimester screen is available between 11-14 weeks gestational age. Depending on the laboratory/hospital, results are usually reported within one week. Results will be reported as a risk assessment for both Down syndrome and trisomy 18. The result could come back as high as a 1 in 2 chance (50% chance the baby has Down syndrome). Alternatively, the risk could be extremely low (ex. 1 in 10,000 chance the baby has Down syndrome). Of course the numbers could fall anywhere between the two extremes. Regardless of the result, it important to remember that this test cannot diagnose or rule out either chromosome abnormality, it’s just providing you with more information.
The test will also be reported as “positive” or “negative.” For example, anyone with a Down syndrome risk greater than a 1 in 270 chance will be considered “positive.” The cut-off between a positive and negative test will vary between labs. Of course, a positive result does not mean the baby has a chromosome abnormality. It simply means that the risk in increased. Here’s an example:
A woman does a first trimester screen and her risk for Down syndrome is 1 in 100 and her risk for trisomy 18 is 1 in 10,000. This would be a negative test for trisomy 18 but a positive test for Down syndrome. A 1 in 100 risk is equivalent to a 1% risk. In other words there is a 99% chance the pregnancy does not have Down syndrome, but because there is an increased risk (above the 1:270 cut-off) this individual will likely be offered additional testing options. (see diagnostics tests and/or non-invasive prenatal testing)
Second Trimester Screening (The Quad Screen)

The obvious difference between the quad screen and the 1st trimester screen is the timing. Another key difference is that along with screening for Down syndrome and trisomy 18 it will also screen for neural tube defects (like spina bifida).
The quad screen is just a blood draw. This test will be looking at four proteins that are made by the pregnancy (AFP, hCG, UE3, DIA). The protein levels will be measured and used to calculate the risk assessment (factors such as maternal age, ethnicity, ect. are again contributory).
The detection rate for Down syndrome is approximately 80% with a false positive rate of 4-5%. The detection rate for trisomy 18 is 80% with a false positive rate of <0.5%. Lastly, the AFP protein is used to screen for open neural tube defects with a detection rate of 80%.
There are multiple other screening options that incorporate combinations/variations of the 1st trimester screen and the quad screen. For detailed sensitivity and specificity of the tests see the table below.
- Full Integrated Screen: This test combines the first trimester screen and the quad screen to increase the overall detection rate for chromosome abnormalities. The main downside is that you won’t receive results until both the first and second trimester test are performed and analyzed.
- Serum Integrated Screen: This test is the same as the full integrated screen except there is no ultrasound as part of the 1st trimester screen (it’s just two separate blood draws). Results will also not be available until both tests are completed. This may be an ideal option for women whose insurance does not cover an ultrasound (or have a limited number of ultrasounds in a pregnancy).
- Sequential Screen: This test is almost the exact same as the full integrated screen. The only difference is that you will receive preliminary results after the first trimester screen. The overall detection rate for Down syndrome is just slightly lower when compared to the full integrated screen.
- Triple Screen: Same test as the quad screen except it analyzes three proteins instead of four (therefore the detection rate is slightly decreased).
- AFP only (second trimester): Having AFP protein measured during the second trimester is a way to screen for open neural tube defects (ONTD). Of course, the AFP protein is already part of integrated screen, sequential screen, and quad screen. Therefore, this test would only be made available to women who only had a 1st trimester screen but would also like to screen for ONTD in the second trimester (80% detection rate). A second trimester targeted ultrasound is another way to screen for ONTD, with an even higher detection rate (see below).
- Second Trimester Targeted Ultrasound: A targeted ultrasound during the second trimester (ideally between 18-20 weeks) is another way to screen for chromosome abnormalities, birth defects, and other signs of genetic disease. The majority of babies that have a chromosome abnormality will also have an abnormal ultrasound finding (possibly multiple findings). Therefore a normal second trimester ultrasound certainly decreases the risk that your baby has a chromosome abnormality or other birth defect. The detection rate for ONTD is approximately 95% (99% for the more severe cases).

Afp4: aka Quad Screen
FPR: False Positive Rate
OAPR: Odds of being Affected giving a Positive Result
(This table is from Integrated Genetics, a LabCorp Specialty Testing Group)
All of the above mentioned tests are prenatal screening tests that should be made available to all women in all pregnancies. In the following sections I will discuss noninvasive prenatal testing and diagnostic testing through CVS or amniocentesis. These tests will not necessarily be discussed or offered to all women. They are generally recommended to be offered to women who are at an increased risk for a chromosome abnormality. Factors that put women in the increased risk category may include advanced maternal age ≥35, ultrasound anomalies, a positive screening test, or a previous pregnancy with chromosome abnormality.
Benefits:
- Screens for Down syndrome, trisomy 18, trisomy 13, and sex chromosome abnormalities at a high detection rate
- May screen for smaller chromosome changes such as microdeletions
- There is not risk to the baby, it’s just a blood draw from the mother
- It can be done as early as week 10 in a pregnancy
Limitations
- Still considered a screening test, although it is highly accurate it is still possible to have a false positive or false negative
- Insurance coverage is sometimes tricky
Noninvasive prenatal testing (NIPT) is a newer technology that is able to isolate fragments placental DNA from a sample of the mothers blood (using cell-free fetal DNA technology). The test is able to screen for Down syndrome, trisomy 18, trisomy 13, and sex chromosome abnormalities with a high detection rate. There are no risks to the mother or the baby because it just involves a blood draw from the mother. The testing can be done as early as week 10 in a pregnancy and results are typically reported within 1-2 weeks.
Even though studies have shown that this testing has high detection rates a low false positive rate, it’s still considered a screening test (not diagnostic). If a person has a positive test it is still recommended to confirm the result through a diagnostic procedure (CVS or amniocentesis). Conversely, a negative test would significantly reduce the likelihood of the pregnancy having a chromosome abnormality but it cannot rule it out. One explanation for a false positive test is whats called confined placental mosaicism (CPM). As I mentioned above, NIPT is testing placental DNA and does not directly test the baby’s DNA. In about 1-3% of pregnancies, the genetics of the placenta can be different from the genetics of the baby. This can lead to false positives (or false negatives).
These tests are often advertised to patients and healthcare providers as >99% accurate. It is important to recognize this is a population-level statistic and only applies to the entire population of women screened. It does not apply to an individual’s result. Since most pregnancies are unaffected and most results are “low risk” or “negative” this test is correct 99% of the time for all women. However, the probability a high risk result indicates an affected fetus is not 99% in the majority of cases. In order to determine how likely a high risk result indicates an affected fetus, you need to know the positive predictive value (PPV). The PPV reflects the chance that a positive test result is a true positive. If you have a positive result, it is important to have a discussion with a genetic counselor or health care provider who is familiar with the PPV of this testing. One helpful resource that can be used is an online NIPT PPV calculator.
Insurance coverage for this test is complicated. As of now most insurances will cover testing for women with certain risk factors. These risk factors may include advanced maternal age ≥35, ultrasound anomalies, a positive screening test, and a previous pregnancy with chromosome abnormality. If insurance is not covering the test and you are paying out of pocket, the cost could be upwards of $1000-$2000 dollars. Some labs will have a policy to limit your out-of-pocket cost.
There are numerous companies that are offering this testing. Each lab is a little different in terms of their detection rates, cost, and how they report results. Here is a list of the labs and the name of their test:
(click on the links to see specific detection rates and other information)
Sequenom: Materniti21 Plus Prenatal Test
Progenity (previously Verinata): Verifi Prenatal Test
Natera: Panorama Prenatal Test
Ariosa Diagnostics: Harmony Prenatal Test
*Important Definitions:
Sensitivity: The number (or percentage) of positive test results that are actually positive. (Ex. A positive test for Down syndrome and the baby actually has Down syndrome)
Specificity: The number (or percentage) of negative test results that are actually negative. (Ex. A negative test for Down syndrome and the baby does not have Down syndrome)
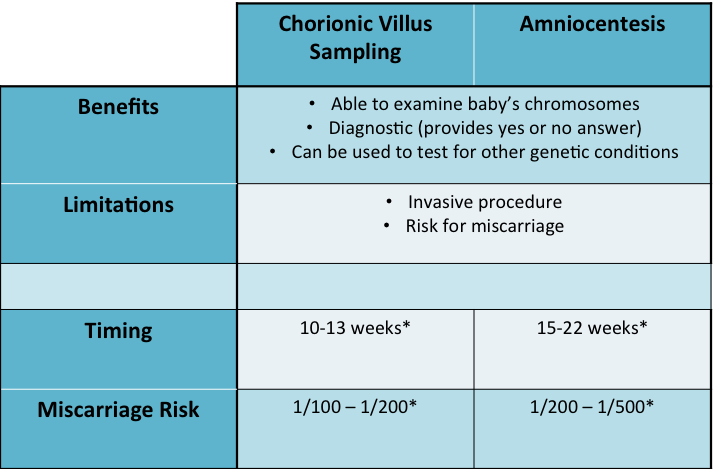
Chorionic villus sampling (CVS) and amniocentesis are the gold standard for prenatal diagnosis. Both usually involve inserting a needle guided by ultrasound through the abdomen of the mother to sample either the chorionic villus (CVS) or amniotic fluid (amniocentesis). These samples contain fetal cells which are then sent to a laboratory. The lab is able to use the sample to get a picture baby’s chromosomes, this is called a karyotype. Next they count the chromosomes to see if there any are extra or missing. This is considered a diagnostic test because in looking at the baby’s chromosomes the lab is able to confirm or rule out a diagnosis of a chromosome abnormality (Down syndrome, trisomy 18, trisomy 13, or any other).
Preliminary results from either test can be reported as early as 24-48 hours after sending in the sample. These preliminary results are done using a technique called fluorescence in situ hybridization (FISH) and just look at certain chromosomes (21, 18, 13, and the sex chromosomes). The FISH analysis is highly accurate (>99%). The final chromosome analysis, in which the full picture of the chromosomes is analyzed, usually takes 1-2 weeks. Most of the time, the final chromosome analysis just confirms what we already knew from the FISH results.
Both the CVS and amniocentesis are considered an invasive test and have an associated risk for miscarriage. The risk for CVS is about 1 in 100 to 200, while the risk for amniocentesis is about 1 in 200 to 400. While this is a low risk it is certainly important to be aware of this before having either test done. The miscarriage risk may vary depending on the hospital or physician.
Another key difference between the CVS and amniocentesis is the timing. The CVS is a first trimester test available between 11-13 weeks gestation while the amniocentesis is a second trimester test that is usually done between 15-22 weeks gestation.
There are also other genetic tests that can be performed from a CVS or amniocentesis sample. One such test is called a microarray. A microarray essentially takes a detailed scan of the chromosomes and looks for any missing or extra pieces that are too small to be visualized on a conventional chromosome analysis. Small pieces of missing or extra chromosome material can sometimes be associated with birth defects, mental retardation, and other medical concerns. However, if a genetic change is found on a microarray analysis it can sometimes be difficult to predict its significance. Some of these genetic changes are benign while others have a more severe impact. An example of a situation in which a microarray might be ordered is for a woman who has multiple ultrasound anomalies. The woman has an amniocentesis to look for a chromosome abnormality and the chromosome studies are normal. She and her doctor decide to order a microarray to look for smaller chromosome changes that could potentially explain the ultrasound findings. Most of the time the test can be done from the amnio sample that was already collected.
Lastly, either a CVS or amniocentesis can be used to test for known genetic conditions in the family. For example, a couple had a previous child with cystic fibrosis and they want to if their current pregnancy also has cystic fibrosis. The couple had genetic testing done after their first pregnancy to find that they were both carriers for a genetic mutation in the cystic fibrosis gene. Therefore, there is a 25% risk to future pregnancies to also have cystic fibrosis. A specific genetic test for cystic fibrosis can be performed on a sample from a CVS or amniocentesis to see if the current pregnancy is affected.